DIY: How to Make Your Own MMS (22.4% Sodium Chlorite Solution) from Scratch
A step-by-step guide to safe and precise home preparation using basic supplies. Links, Recipes, and FAQs
Note: This is for educational purposes. This is not medical advice, and I am not telling you what you should do. Every person is or should be in control of their own health in spite of what the current medical establishment would like you to believe.
Introduction
Making sodium chlorite solution (commonly referred to as MMS) is foundational for chlorine dioxide protocols. This article and video explain the rationale, essential supplies, and detailed, step-by-step instructions. The end product is a carefully measured and filtered sodium chlorite solution, ready for activation. In the next article, we will go over making the acid activator.
Sodium chlorite typically comes in flake form with 80% purity. The goal is to create a 22.4% solution, which is the industry standard for protocols and dropwise mixing with acid activators. Following precise measurements and safe handling ensures the solution is both effective and safe for storage and later use. There will be a question and answer at the end of this article that will hopefully answer the questions that are arising in your mind as you read this.
Supplies Needed
Sodium chlorite flakes, 80% pure. Suppliers of sodium chlorite flakes: Supplier 1, Supplier 2, Supplier 3. At any one of those suppliers or Amazon search the term “Sodium Chlorite Flakes 80%.”
Distilled water
Digital scale (grams)
Glass mixing bowls (one small for weighing, one large for mixing)
Mixing spoon
Unbleached coffee filter (optional for sediment)
Amber glass bottle (for storage). Suppliers of amber glass dropper bottles: Supplier 1, Supplier 2, Supplier 3
Labels and marker
Important: Only use glass and plastic for measuring and mixing—avoid metal utensils and containers.
Step-by-Step Construction
Preparation
Set Up Workspace: Ensure the working area is clean and clear.
Gather Materials: Verify that all supplies are present and the digital scale has working batteries.
Warm Water (Optional): If desired, gently warm distilled water to around 120°F (49°C) to speed up the dissolving of sodium chlorite.
Weighing
Tare the Scale: Place the empty glass bowl on the scale and zero it (tare).
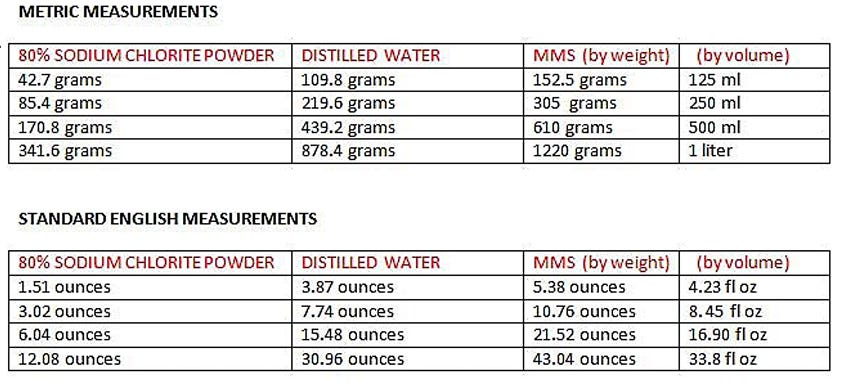
Measure Sodium Chlorite: Weigh out the desired amount (e.g., 280 grams for a batch making 850 ml, or scale to 100 grams for smaller test batches).
Measure Distilled Water: For 80% sodium chlorite, use the proportion 280g sodium chlorite to 720g distilled water for 850 ml. For 100g sodium chlorite, use 257g distilled water.
To make smaller or larger amounts, use the chart below to determine amounts of sodium chlorite and distilled water.
Mixing
Combine: Place weighed sodium chlorite into the large glass mixing bowl.
Add Water: Pour in the appropriate amount of distilled water.
Stir to Dissolve: Mix until fully dissolved. This may take up to 30 minutes, especially if water is not pre-warmed.
Filtration (Optional)
Filter Solution: If sediment remains, pour the solution through an unbleached coffee filter into a clean container.
Storage
Pour and Label: Transfer the finished solution into an amber glass bottle, seal, and label “Sodium Chlorite 22.4% - [Date]”.
Store Safely: Keep the bottle out of sunlight, in a cool, dark location.
Key Notes
Avoid metal and utensils when handling sodium chlorite solution.
Ensure labels are clear to prevent accidental misuse.
Use calculators or the provided lazy charts for scaling batches to maintain 22.4% concentration.
Always store in opaque amber bottles to minimize degradation from light.
Note: In the video when talking about the distilled water, I inadvertently stated 720 ounces of distilled water and I should have said 720 mL of distilled water. 720 g of distilled water is equivalent to 720 mL of distilled water.
In the video, I mentioned using plastic bottles. I no longer recommend plastic bottles because when storing for longer than one year I have seen plastic degradation in all of the different kinds of plastic bottles I have used.
Frequently Asked Questions
Q: Why should I bother making my own solutions?
A: There are several reasons:
You will know your solutions have been made properly.
You will save a lot of money. The cost of making your own is approximately one-tenth the cost of buying pre-made solutions.
You will not be dependent on the manufacturers of pre-made solutions.
If there are others around you that need help, you will have plenty to share.
80% sodium chlorite powder can store for 25+ years if stored properly. The solutions las 2-5 years in good conditions.
Q: Why are the sodium chlorite flakes only 80%?
A: This is a safety precaution. When manufacturers produce the sodium chlorite, it is in a liquid state and must be dried to obtain the sodium chlorite flakes. They dilute the sodium chlorite to 80% using sodium chloride (salt water) to prevent spontaneous combustion during the drying process.
Q: How long will the liquid solution store for?
A: If stored in a cool, dry, and light free location, the solutions should easily store for 2 years minimum. I have had some people tell me that they have tested solutions that were over five years old and they still had good activation. Make sure you always test before throwing out. Add three drops of acid activator to three drops of the sodium chlorite and see if you get the typical color change to yellow/amber and the distinct chlorine like smell. If possible use an acid activator that is new so that you only have one variable to deal with.
Q: Can I store the 22.4% sodium chlorite solution in plastic bottles?
A: I do not any longer recommend storing in plastic bottles. I have tried storing in different kinds of plastic bottles and when storing for LONG periods of time, all forms of plastic that I have tested have degraded. Glass is best.
Q: Are the rubber tips on the glass dropper bottle lids safe.
A: Yes, the sodium chlorite will not be in direct constant contact with the rubber stopper that is used on the dropper and this is fine. For a very long-term storage of greater than two years, regular (non-dropper) caps are recommended due to degradation of rubber from normal exposure to oxygen in the air.
Q: Why can't I use a metal spoon to stir?
A: Sodium chlorite will react with metal and metal ions can go into the solution.
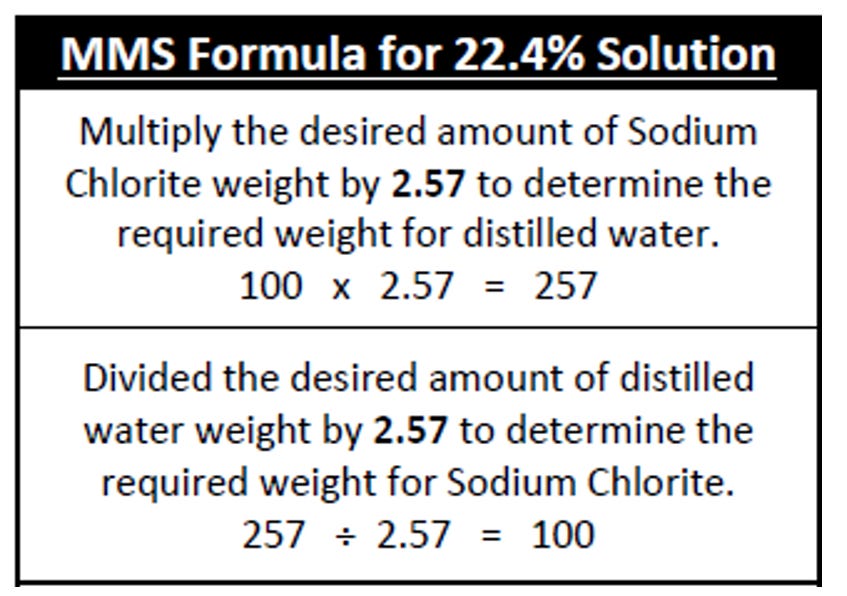
Q: If I have only a specific amount of sodium chlorite and want to use all of it how can I calculate how much distilled water needs to be added?
A: The formula below can be used to calculate the amount of distilled water that needs to be added to a specific amount (by weight) of sodium chlorite to get a 22.4% solution. In the example below, if we have 100 grams of sodium chlorite. (example: for 22.4% solution, for 100 grams sodium chlorite, use 257 grams of distilled water)
In the chart below, the top formula is used if you have a specific amount of sodium chlorite powder you want to use. The bottom formula is used if you have a specific amount of distilled water and want to know how much sodium chlorite flake you need.
Q: Are there any calculators that I can use to make sure my calculations are correct?
A: Yes, here is a downloadable Excel file that has a built-in calculator for the sodium chloride and also for the HCl acid activator if needed. Calculator Download
If you've read through all of these frequently asked questions and still have a question, please feel free to ask in the comments below.
Here is a link to the printable PDF that I mentioned in the video: What is MMS and How to Make It



I appreciate your time and dedication with sharing this.
Thank you very much for such detailed information but am wondering if you might have time to describe what it’s used are for? I believe it is grest in removing harmful toxins from the body but more clarity would be grest, if possible, thanks again